5 Explosive Prime Editing Cystic Fibrosis Breakthroughs Promise Amazing Cures
On This Page
The landscape of genetic medicine is undergoing a radical transformation, driven by innovations that once seemed confined to science fiction. Among these, Prime Editing stands out as a revolutionary “search-and-replace” technology for the genome. This incredible precision tool is now demonstrating breathtaking potential, especially in the fight against genetic disorders like cystic fibrosis (CF).
For millions affected globally, a future where genetic diseases are not just managed but cured is becoming increasingly tangible. We’re about to delve into 5 brilliant ways Prime Editing Cystic Fibrosis research is not just advancing, but fundamentally changing the paradigm for this devastating condition.
Understanding Prime Editing The Precision Tool for Cystic Fibrosis
Before we explore its direct applications, let’s unpack Prime Editing itself. Developed by David Liu and his team at the Broad Institute, Prime Editing is often described as a next-generation genome editing tool, building upon the foundations of CRISPR-Cas9 but offering unparalleled precision. While CRISPR acts like a pair of molecular scissors, creating double-strand breaks that can lead to unpredictable insertions or deletions (indels), Prime Editing is more akin to a word processor’s find-and-replace function.
It utilizes a Cas9 nickase (which cuts only one DNA strand) fused to a reverse transcriptase enzyme, guided by a prime editing guide RNA (pegRNA). This pegRNA not only directs the editor to the target site but also carries the template for the desired new genetic sequence. Data indicates this method significantly reduces off-target edits and minimizes unwanted indel formation, with studies reporting indel rates as low as 0.001% compared to conventional CRISPR’s 1% to 10%. This level of accuracy is critical, particularly when considering permanent genetic modifications for diseases like cystic fibrosis.
Directly Correcting the CFTR F508del Mutation
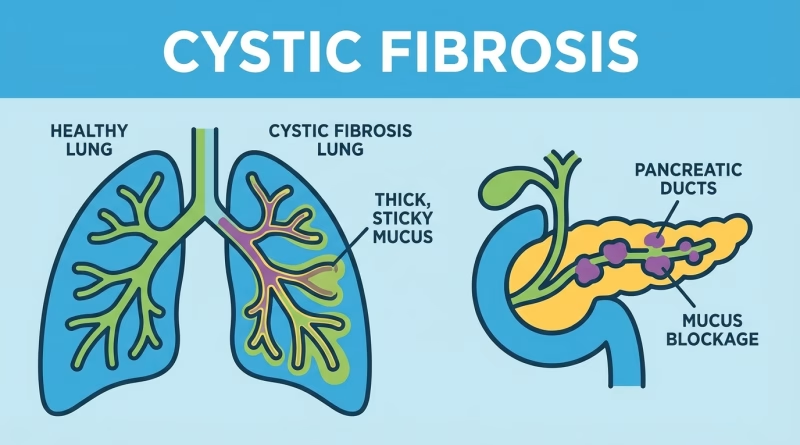
Cystic fibrosis is predominantly caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, which provides instructions for making a protein that regulates the flow of salt and water in and out of cells. The most common mutation, accounting for approximately 70% of all CF cases, is F508del – a deletion of three base pairs that leads to a misfolded protein and impaired chloride channel function. Historically, treatments have focused on managing symptoms or improving CFTR protein function, but not correcting the underlying genetic error.
This is where Prime Editing offers a monumental shift. By designing a specific pegRNA, scientists can direct the prime editor to the exact F508del site in the CFTR gene. The reverse transcriptase then uses the pegRNA’s template to seamlessly insert the missing three base pairs, effectively reversing the mutation at its source. Preclinical studies have shown promising results, with Prime Editing successfully restoring wild-type CFTR sequence in patient-derived organoids and cell lines, leading to measurable improvements in chloride transport activity. For example, some studies reported functional restoration of CFTR activity by as much as 30-50% in edited cells, a range considered clinically significant for patient benefit.
Expanding Beyond F508del Addressing Rare Cystic Fibrosis Mutations
While F508del is the most prevalent, over 2,000 different mutations in the CFTR gene have been identified, many of which are rare and do not respond to existing mutation-specific drugs. This presents a significant challenge for a large subset of the cystic fibrosis population. The beauty of Prime Editing lies in its versatility. Unlike gene replacement therapies that introduce an entirely new gene, or base editors that are limited to specific base pair conversions (e.g., C to T, A to G), Prime Editing can precisely install virtually any single-nucleotide change, small insertions, or small deletions.
This broad applicability means that pegRNAs can be designed to target nearly any pathogenic CFTR mutation, whether it’s a point mutation causing premature stop codons (e.g., G542X), splicing defects, or other small indels. This capability dramatically expands the addressable patient population, potentially offering a curative strategy for individuals with ultra-rare CFTR mutations who currently have no targeted therapies available. This adaptability marks a significant leap towards truly personalized genetic medicine for cystic fibrosis.
Delivering the Cure In Vivo Challenges and Advancements
Achieving a therapeutic effect with Prime Editing in a living organism, particularly for a systemic disease like cystic fibrosis that primarily affects the lungs, requires efficient and safe delivery of the editing machinery to target cells. This presents a significant hurdle. Early research focuses on two primary delivery methods: adeno-associated virus (AAV) vectors and lipid nanoparticles (LNPs).
AAV vectors are highly effective at delivering genetic material to lung cells but can be limited by package size and potential immune responses. Recent advancements in AAV serotype engineering are showing promise in overcoming these limitations, improving tropism for lung epithelial cells while reducing immunogenicity. Lipid nanoparticles, on the other hand, are gaining traction due to their ability to encapsulate larger payloads and their lower immunogenicity compared to viral vectors. Researchers are actively optimizing LNPs for targeted delivery to lung tissue, with studies reporting efficient delivery of mRNA-encoded prime editors to respiratory cells. The challenge remains to achieve sufficient editing efficiency in a large enough proportion of cells in the lungs, pancreas, and other affected organs to restore physiological function. However, the rapid progress in both viral and non-viral delivery systems is a testament to the scientific community’s commitment to bringing Prime Editing Cystic Fibrosis treatments to the clinic.
The Promise of a One-Time Genetic Fix for Cystic Fibrosis
Perhaps the most revolutionary aspect of Prime Editing is its potential to offer a permanent, one-time cure rather than symptomatic management. Current treatments for cystic fibrosis, while vastly improved, require lifelong adherence to complex regimens of medications, physiotherapy, and hospital visits. The financial burden is substantial, with annual costs for some CFTR modulators exceeding $300,000 per patient, alongside the immense physical and emotional toll on individuals and families.
By precisely correcting the underlying genetic defect, Prime Editing aims to restore normal CFTR protein function, potentially eliminating the need for continuous intervention. Imagine the transformative impact on quality of life, freeing patients from the daily struggle of managing their disease. While the long-term durability of prime-edited cells, especially in rapidly regenerating tissues like the gut and airways, is an area of ongoing research, the prospect of installing a permanent genetic repair within a significant percentage of affected cells offers an unprecedented path to truly arresting disease progression and potentially reversing damage. This shift from managing a chronic illness to curing a genetic disease represents an amazing paradigm shift.